![]()
Electrochemical Reactivity and Electrosynthesis of Heteroelement Compounds
Electrochemical Reactivity
Electrochemical Methodology
Electrogenerated Reagents
Redox Chemistry of Metallatranes
Silyl Radicals
Electrosynthesis
|
|
|
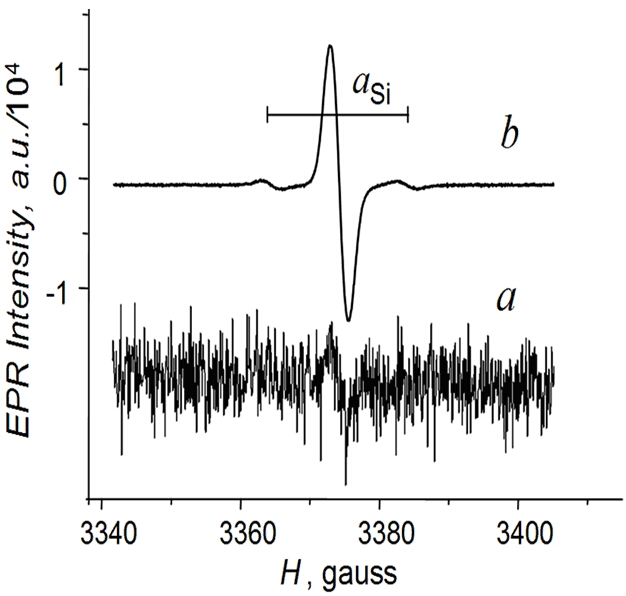 |
The most surprising result was that these bonds can consecutively give away or accommodate two electrons and this without the cleavage of the M-M linkage! Diode-like behavior of these systems due to mixing the electrons of M=M double bonds with n-electrons of S was demonstrated. |
|
|
||
|
Depending on donor-acceptor interactions through space, easily modulated by polarity of the
reaction media, products of different nature were obtained from the oxidation of
arylalkylselenides. So simple change of proportions of the components of a binary solvent
directs the process to either allylic or a-Nu-substituted products. |
||
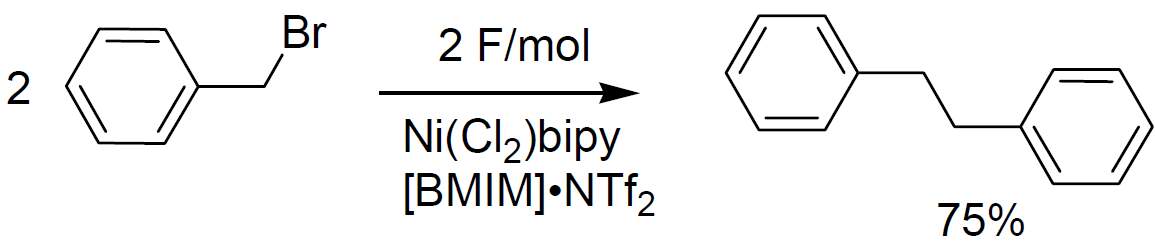
|
Cathodic Ni(II) cathalysed formation of biaryls and bibenzyls from the corresponding organohalides was shown to be easily and selectively realizable in neat ionic liquids, with no co-solvent added at all. |
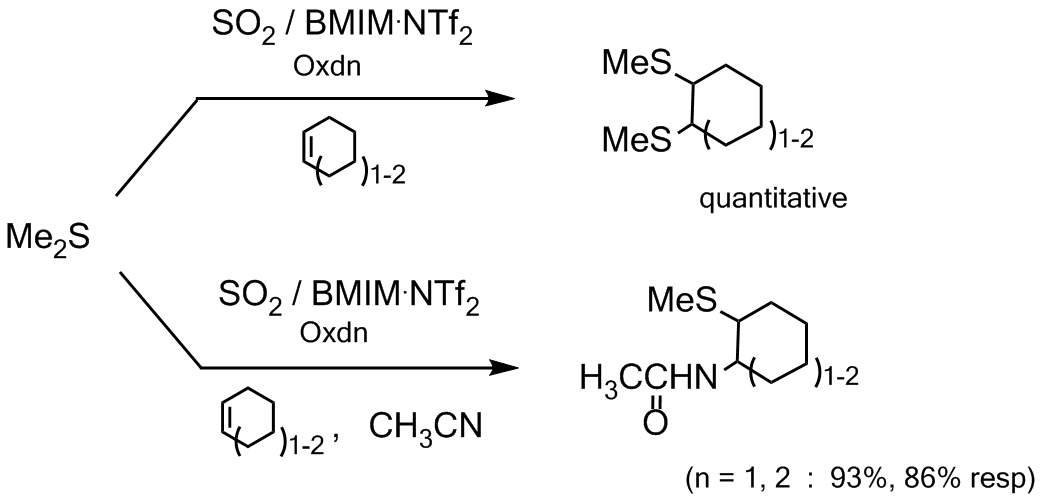 |
Addition of small amount of liquid sulfur dioxide to BMIM NTf2 ionic liquid dramatically decreases the viscosity of this media and allows combining its low nucleophilicity with a good processability and recycling use, the latter is rather easy in this case because the SO2 is a gas at room temperature and all is needed is just to let it evaporate. |
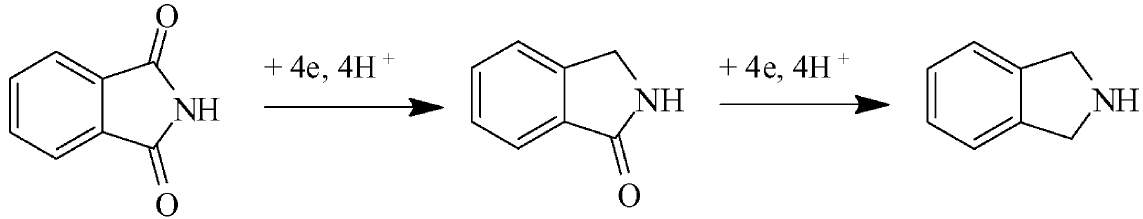 Cathodic decarbonylation of phthalimide is a very convenient way for
preparing isoindoline, the added value of this process is more that 1000
times. The tricky thing here is to master the interplay of hydrogen
evolution and that of the target process. Cathodic decarbonylation of phthalimide is a very convenient way for
preparing isoindoline, the added value of this process is more that 1000
times. The tricky thing here is to master the interplay of hydrogen
evolution and that of the target process.
|
|